They are manufactured under a Quality Management System compliant with the standards PN-EN ISO 13485:2016–04, PN-EN ISO 9001:2015, and the medical directive 93/42/EEC, and are registered with the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
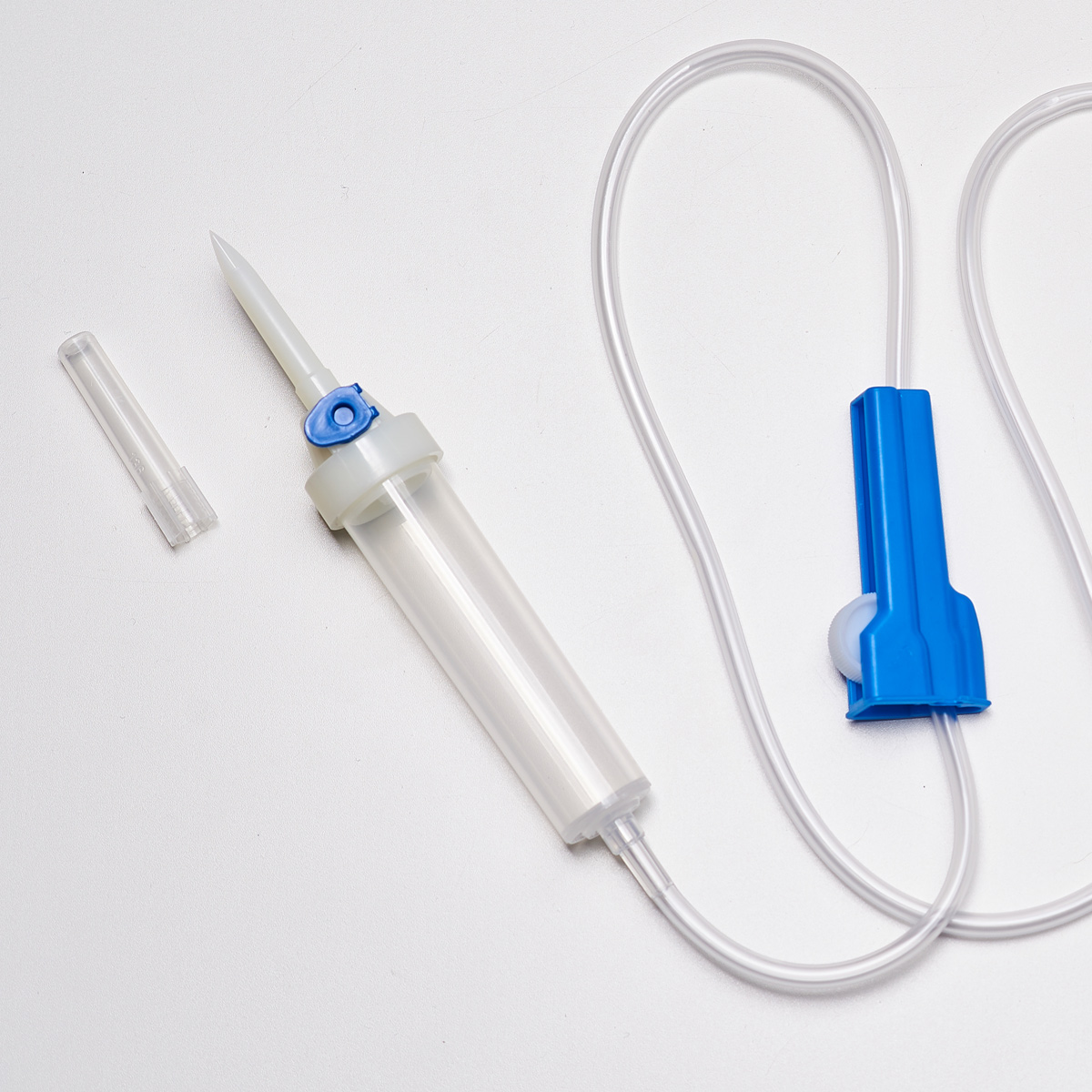
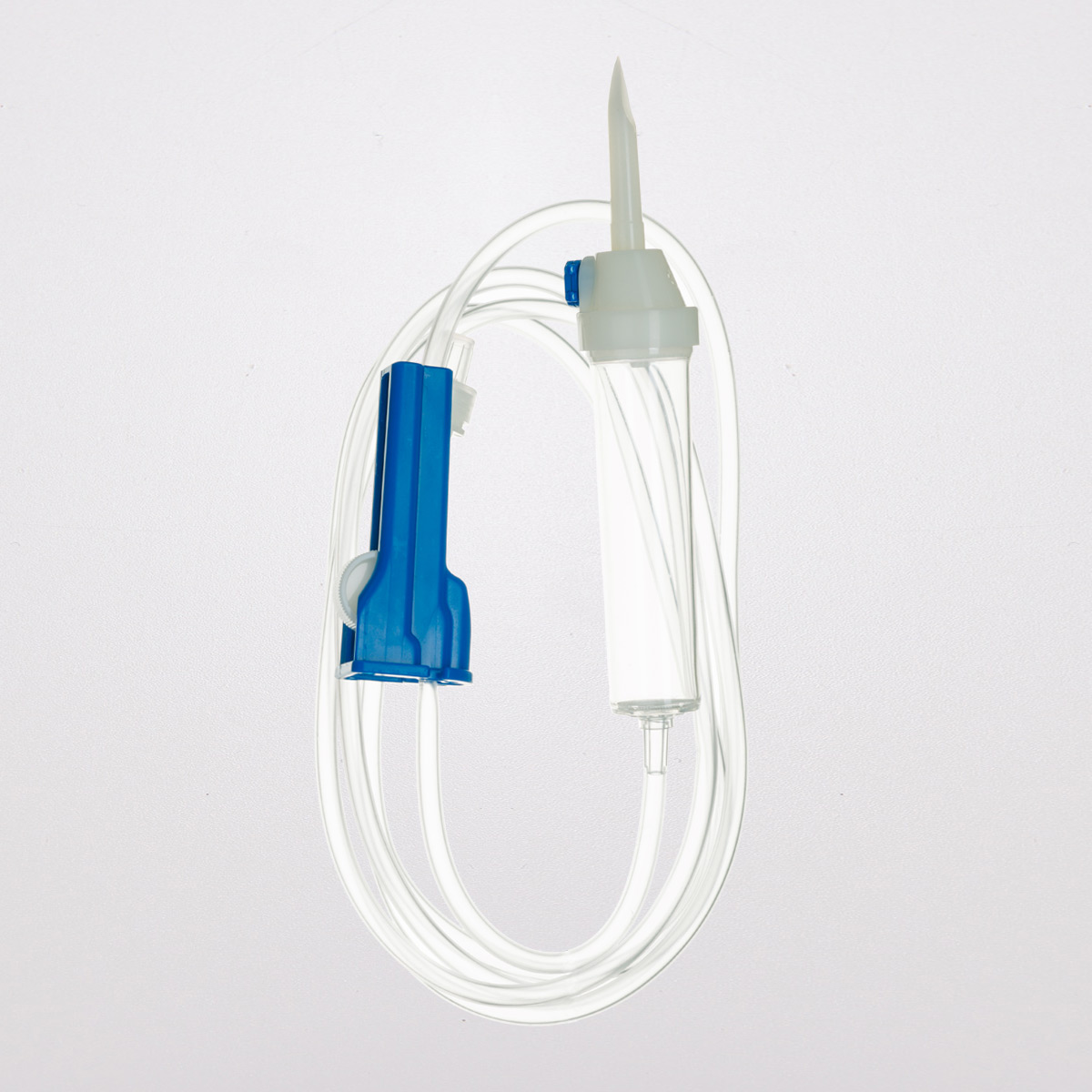
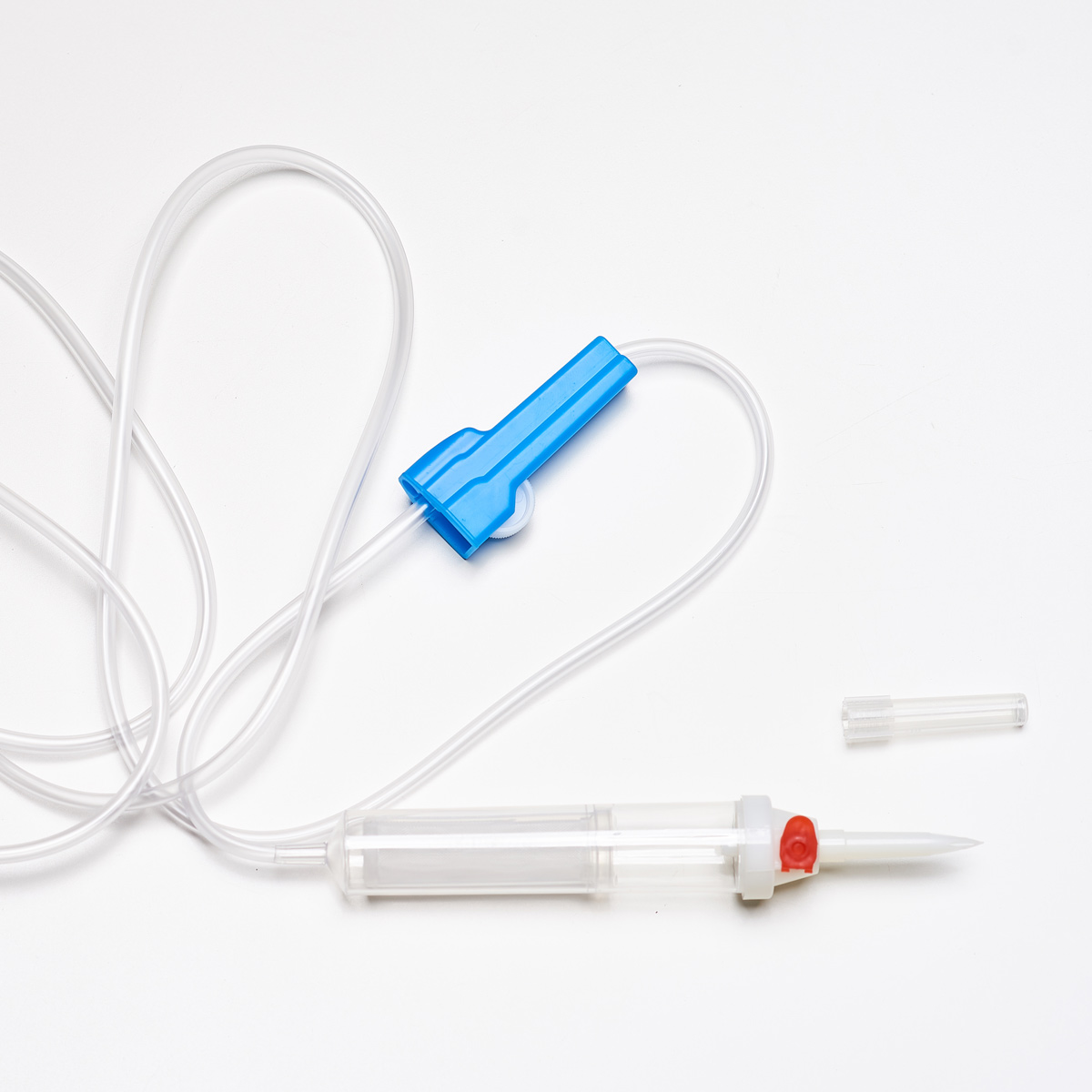
The infusion fluid transfer sets consist of the following elements:
- Needle cover with longitudinal ribs for easy removal
- Dual-channel needle to prevent the set from falling out of bottles and fluid from leaking at the connection point; easy insertion even into small soft containers
- Drip chamber (20 drops = 1 ml ± 0.1 ml)
- Luer-lock connector cover
- Luer-lock cone connector
- 150 cm medical-grade tubing made from PVC free of DEHP, BBP, DBP
- Hydrophobic air filter
- PVC-free drip chamber
- Fluid filter with 15 µm pore size
- Blue on/off cap for the hydrophobic filter
- Additional injection connector
- Roller clamp with the manufacturer’s name
- Dedicated holder for the needle after use
- Tubing clip
The infusion fluid transfer sets are:
- Packaged in individual paper-foil packaging
- Packed in boxes of 180 sets
 Polski
Polski